Understanding ADPKD & PROGRESSION
Rapidly progressing ADPKD vs being at risk for rapidly progressing ADPKD
The truth about autosomal dominant polycystic kidney disease (ADPKD) progression is that it looks different for everyone—even within the same family
- A parent with ADPKD has a 50% chance of passing it on to each child
- It is important to know that ADPKD disease progression and symptoms vary amongst patients
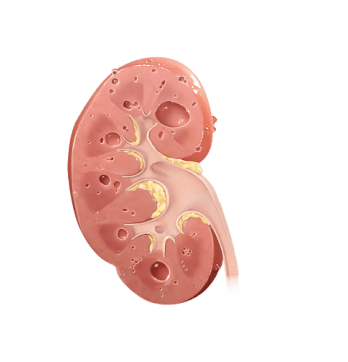
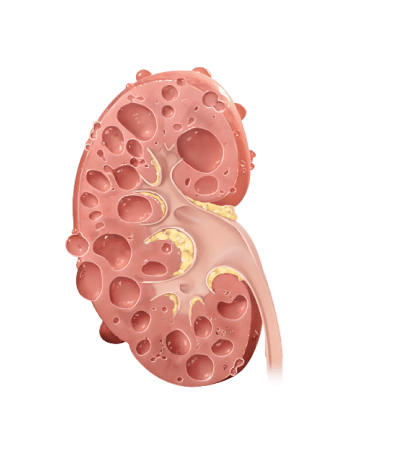
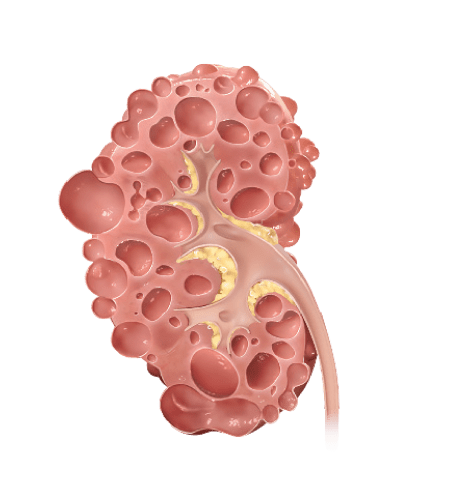
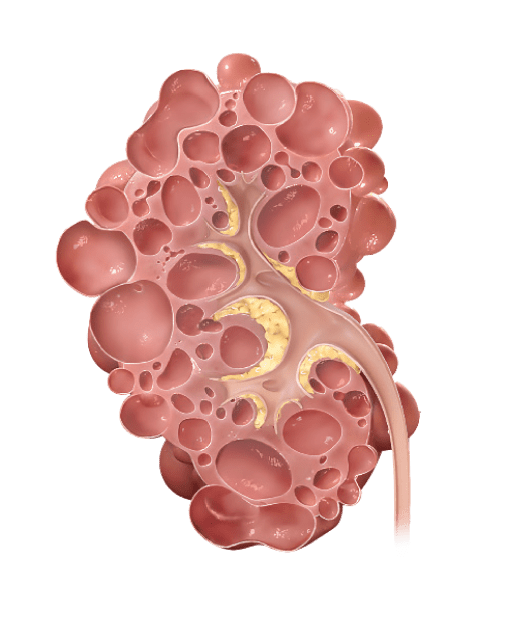
With rapidly progressing ADPKD, kidney function worsens and cysts grow more quickly, leading to early kidney failure
- According to the Mayo Clinic ADPKD study, out of 538 patients, ~2/3 of this group were at risk for rapid progression
Why does kidney size matter?
In ADPKD, kidney growth caused by cysts often happens before your kidney function decline appears. Kidney function, measured by estimated glomerular filtration rate (eGFR), may remain stable while your kidneys continue to grow and can mask the irreversible damage. Because of this, kidney size, or total kidney volume (TKV), is the best predictor of ADPKD progression.
Your nephrologist can use an ultrasound, magnetic resonance imaging (MRI), or computed tomography (CT) to measure your kidney size and determine your risk of ADPKD progression.
Assessing your ADPKD progression
Is your eGFR rapidly declining?
Your ADPKD could already be rapidly progressing.
A person with ADPKD is considered to be already rapidly progressing when their eGFR (a test result that measures how well the kidneys work) declines quickly.
eGFR is declining quickly when it drops: ≥ 3 mL/min/1.73 m2 per year over a 3-5 year period.
Is your eGFR stable?
You could still be at risk of rapidly progressing ADPKD.
There are a number of factors that may put someone already living with ADPKD at risk of rapid ADPKD progression. These may include:
Be Proactive
Wherever you are in your ADPKD journey, it's important to talk with your nephrologist to develop an appropriate treatment plan for your individual situation.
The sooner you start, the sooner you can help take control of your ADPKD and may start to experience the benefits of slowing down your disease progression. Don't wait, take the first step today. Make a plan to have a discussion about what you can do to help manage your ADPKD at your next appointment.